1. Noble gases, why so stable?
Chemistry is the study of reactions. It’s also the study of non-reaction. Why some atoms don’t react to form any bond with another atom?

Atoms of noble gases do not usually react and combine with other atoms. They remain as individual atoms. There seems to be a je ne sais quoi about their electronic configuration: the number of valence electrons (which are also called outermost electrons).
2. If you can’t beat them, join them: two atoms form a covalent bond by sharing electrons

Unlike the noble gas helium, a hydrogen atom is highly unstable. On its own, it reacts and combines with another hydrogen atom. This is why hydrogen exists as diatomic molecules.
But hang on, what does it mean by unstable? Let’s explore this in terms of electrons.

A hydrogen atom is unstable precisely because it is short of one electron as compared to the noble gas helium, which has two valence electrons. To put it in professional parlance, a hydrogen atom does not have the electronic configuration of a noble gas.
To achieve it, two hydrogen atoms share their two electrons. The type of bond formed here is called covalent bond, because atoms co-own valence electrons.
A covalent bond is formed by the sharing of a pair of electrons in order to gain the electronic configuration of a noble gas.
3. Other non-metallic elements form covalent bond too

Let’s move down the periodic table to look at the second period with the noble gas neon. Take a deep breath.
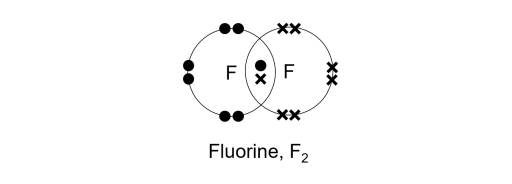
Like hydrogen, a fluorine atom is short of one electron to achieve the noble gas configuration of neon, a noble gas. To achieve it, two fluorine atoms will contribute an electron each, sharing a net total of one pair of electrons. Voila, a single covalent bond is formed!
The shared pair of electrons is co-owned by both atoms. In other words, each atom will have 2 shared electrons and 6 non-bonding electrons, giving them 8 valence electrons each. Quick math!
4. Get more electrons by forming double covalent bond and triple covalent bond

Referring back to the periodic table, as we move left from fluorine to oxygen, the electron deficit increases. An oxygen needs two more electrons! So two oxygen atoms will share two pairs of electrons, forming a double covalent bond. This means that each atom has 4 shared electrons and 4 non-bonding electrons, like in the noble gas neon.
As we move further left to nitrogen, the electron deficit increases to three. Two nitrogen atoms will band together, sharing three pairs of electrons in a triple covalent bond. This will give them 6 shared electrons and 2 non-bonding electrons each. Once again, order is restored; and noble gas electronic configuration, achieved!
5. Different non-metallic elements form compounds via covalent bond
Any combination of non-metallic elements can form covalent bonds by sharing electrons. This forms a covalent compound. In the compound, each atom has the electronic configuration of a noble gas.

A fluorine atom can achieve the noble gas electronic configuration by forming a single covalent bond with hydrogen. And so, the compound hydrogen fluoride is formed.

Likewise, a nitrogen atom can bond covalently with hydrogen. However, nitrogen atom has a bigger electron appetite. With 5 valence electrons, it needs 3 more electrons to achieve the noble gas electronic configuration.
Therefore, a nitrogen atom will recruit 3 hydrogen atoms, forming 3 single covalent bonds in the compound ammonia.
6. Cheem word clarification: valency
We know that valence electrons refer to outermost electrons, and covalent as co-sharing valence electrons. But what about valency that we always hear in class but is not quite explained?
Valency is the maximum number of hydrogen atoms that may combine with the atom of an element under consideration.
IUPAC, 1996
| Non-metal | Group | No. of valence electrons | Valency |
|---|---|---|---|
| Hydrogen | I | 1 | 1 |
| Carbon | IV | 4 | 4 |
| Nitrogen | V | 5 | 3 |
| Oxygen | VI | 6 | 2 |
| Fluorine | VII | 7 | 1 |




