1. Heat it up to speed it up: increasing temperature
The hot afternoon heat is punishing. Our brain rots and stops working in afternoon classes. Food goes bad faster outside the fridge. While the former is an exaggeration (for most of us), the latter is an example of a chemical reaction. Its reaction speed is greater when the temperature is higher.
But why? To answer the big question of why reaction speeds up when temperature rises, we have to think small. Let’s consider what happens to the reacting particles during a reaction.

To react, particles must first bump into each other. Yet, like how some fist bumps are more awkward than successful, some particle bumps fail to produce any chemical change. That’s because there is another criterion. Crucially, the colliding particles also need to have sufficient energy to overcome the activation energy.
It is only when particles collide and have sufficient energy that they react to form the products. We call these energetic rendezvous effective collisions.
Therefore, to increase reaction speed is to increase the frequency of effective collisions.
When temperature increases, particles move faster and have greater energy. This increases the frequency of effective collisions to increase reaction speed.
2. The opposite of social distancing: increasing concentration or pressure to increase reaction speed

Another way to increase the frequency of effective collision is to crowd the same space with ever more reacting particles. This is when:
- Concentration of solution increases, whereby there are more solutes per unit volume
- Pressure of gas increases, whereby there are more gaseous particles per unit volume
When concentration or pressure increases, there are more particles more unit volume. This increases the frequency of effective collisions to increase reaction speed.
3. Divide and conquer: decreasing particle size to increase reaction speed
When reactants are in different physical states that do not mix, the reaction only happens at the surface where the two reactants are in contact.

Let’s take the reaction between solid copper(II) oxide and aqueous sulfuric acid as an example. When we halve the copper(II) oxide particle, we create two new contact surfaces where the knife went through.
Generally, when we reduce the particle size by finely dividing, grinding or pulverising a solid, we increase the surface area. This makes effective collisions more frequent at the more extensive surface.
When particle size decreases, there is a greater surface area of contact. This increases the frequency of effective collision to increase reaction speed.
4. Pro gamer move: dropping a catalyst
A catalyst is another substance that we can add to a reaction mixture to speed up the reaction. It only affects how the reaction proceeds, but not what products are formed.
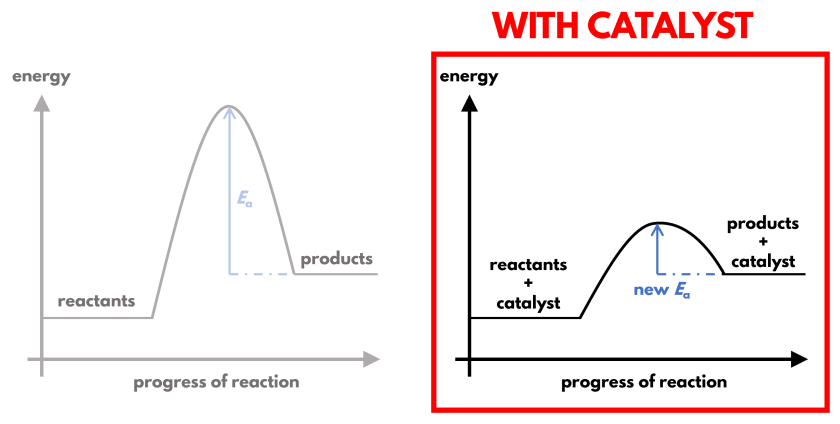
By changing how the reaction proceeds, the activation energy is now lower. This is shown in the energy profile diagrams above, whereby the ‘hump’ that represents the activation energy is gentler and lower with a catalyst.
With a reduced activation energy barrier, a greater proportion of reactants have sufficient energy to overcome it. This increases the proportion of effective collisions without changing the total collision frequency nor the collision energy of the reactants.
Interestingly, catalysts remain unchanged at the end of the reaction. This means that a small amount of catalysts can process and speed up the reaction of a large amount of reactants.
Enzymes are Catalysts Found in Cells
In humans and other living things, our cells contain catalysts too! However, they are made up of large molecules called proteins. We call these protein-based biological catalysts enzymes.
Catalysts and enzymes provide an alternative reaction pathway with a lower activation energy, which more colliding particles have sufficient energy to overcome. This increases the frequency of effective collision to increase reaction speed.
5. Slow it down by doing the opposite
We have talked about the five ways to speed up a reaction. The contrary is true. To slow down a reaction, simply do the opposite:
- Decreasing temperature
- Decreasing concentration (of a solution)
- Decreasing pressure (of a gas)
- Increasing particle size (of a solid)
- Removing the catalyst




